Table of Contents
The ultimate nursing guide to sepsis Written by Matthew Javni – ICU Critical Care Nurse, Melbourne Australia
It is important to note that this document does not serve as a substitute for your organization’s policies and procedures. This is intended for educational purposes and combines evidence drawn from current literature and clinical experiences. While The Nurse Break uses reasonable endeavours to ensure that all content is complete and accurate at the time of publication we accept no responsibility for any error, omission or defects contained in the content.
Read other clinical articles here
What is Sepsis?
The Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock (2016) defines sepsis as “life threatening organ dysfunction caused by a dysregulated host response to infection”. This differs from septic shock, which is characterised by the circulatory collapse and metabolic dysfunction that may ensue when the body mounts a dysregulated immune response to a large enough scale. To better contextualise sepsis as a dysregulated response, it is helpful to review the normal physiological response to microbial invasion; consider the process of wound healing.
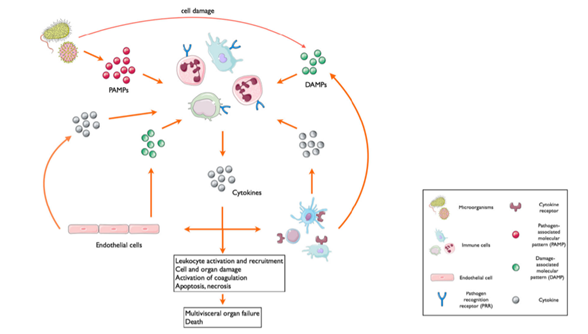
The first stage of wound healing is the inflammatory phase (Li et al., 2007). Immediately following injury, various leukocytes are recruited via their pattern recognition receptors which identify damage-associated molecular patterns (DAMPs) to initiate inflammatory processes in the absence of an invading pathogen (Chousterman et al., 2017). This process is then compounded following microbial invasion when the same pattern recognition receptors bind with pathogen-associated molecular patterns (PAMPs) located on the invading pathogen (Chousterman et al., 2017). In order to target damaged tissues, recruited leukocytes extravasate from nearby capillaries into the wound bed by way of vasoactive mediators such as histamines, bradykinins, and free radicals, which are largely triggered by complement activation, mast cell degranulation and cytokine release (Li et al., 2007).
The inflammatory phase of wound healing requires a delicately balanced combination of pro-inflammatory and anti-inflammatory mediators, which augment localised vessel permeability and vasodilation to expedite the delivery of white blood cells to vulnerable tissues (Chousterman et al., 2017; Li et al., 2007). While the multiple complex and overlapping immune signalling cascades go beyond this rudimentary summary of innate immunity, it demonstrates how a well-orchestrated, localised immune response could become catastrophic if the scales were disproportionately tipped in favour of proinflammation.
A widespread, systemic inflammatory response occurs when damage-associated (as opposed to pathogen associated) molecular patterns continue to be exposed as the result of immune-mediated cell death (Chousterman et al., 2017). This auto-amplifying process (see figure on next page) leads to a large-scale cytokine release, known as a cytokine storm (Chousterman et al., 2017). The now overwhelming concentration of pro-inflammatory cytokines means that the previously localised coagulation, vasodilation and increased capillary permeability occurs globally; resulting in the diffuse intravascular coagulation, gross vasoplegia and widespread fluid shifting that is characteristic of sepsis (Chousterman et al., 2017).
Recognising sepsis in clinical practice
Sepsis screening tools promote early recognition of sepsis and initiation of treatment resulting in a demonstrated mortality benefit (Rhodes et al., 2017). Despite this proven mortality benefit, there is a deficit in current literature as to which trigger warning system yields the best result. As such, current guidelines have abandoned the well-known systemic inflammatory response syndrome (SIRS) criteria as the preferred tool for identifying sepsis without recommending a supplementary risk assessment, leaving it to individual organisations to decide within their own policy development (Rhodes et al., 2017). Former international guidelines advised that a patient could be clinically identified as septic if they met two or more of the SIRS criteria (below) with a suspected or confirmed source of infection, and many organisations have continued this practice (Marik & Taeb, 2017). Other tigger warning tools often adopted by healthcare services include the sequential organ failure assessment (SOFA) or quick sequential organ failure assessment (qSOFA) scores, and the national early warning score (NEWS) or modified early warning score (MEWS; the George Institute for Global Health, 2021).
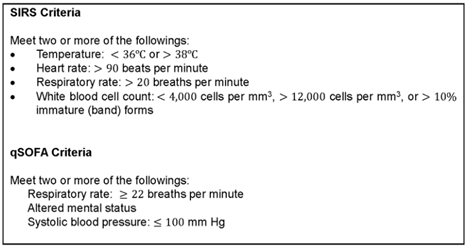
One of the main challenges in recommending a universal warning system is the difference in sensitivity between different trigger warning tools across different healthcare settings. For example, a systematic review conducted by the George Institute for Global Health (2021) found that the SIRS criteria and qSOFA + lactate (LqSOFA) scores had high specificity for screening patients presenting to the Emergency Department. Conversely, the NEWS and MEWS trigger warning scores may have an advantage in acute in-patient settings for recognising deteriorating patients but were more complex and have not been directly compared to former strategies (the George Institute for Global Health, 2021).
As it stands, the 2021 report from the Australian Commission on Safety and Quality in Health Care recommends use of the LqSOFA score since the measurement of lactate improves the sensitivity of scoring systems, while recognising the need for continued research in this field (the George Institute for Global Health, 2021). Regardless of which specific tool has been recommended in your local policies and procedures, there are overlapping clinical red flags which indicate that a patient may be experiencing sepsis which include (Cawcutt & Peters, 2014):
- Fever or hypothermia indicating that an immune / inflammatory response has been mounted
- Tachypnoea as the body enters anaerobic metabolism due to increased metabolic demand and/or inadequate perfusion. The respiratory drive increases to “breathe off” carbon dioxide in compensation for lactate production via the carbonate/carbonic acid buffer system.
- Hypoxia/hypoxaemia as cellular oxygen demand increases, the rate of oxygen extraction also increases. Cellular hypoxia may be exacerbated by biochemical derangements such as metabolic acidosis and high core body temperature which create a right shift in the infamous oxyhaemoglobin dissociation curve. This means that oxygen has a lower affinity for haemoglobin, the primary transport protein for tissue oxygen delivery. As such, shifts these metabolic derangements act as both a catalyst and consequence of cellular hypoxia, as the subsequent shift in the oxyhaemoglobin dissociation curve results in worsened oxygen delivery to target tissues.
- Tachycardia as the body enters a poor-perfusion state and metabolic demand increases, inadequate oxygen delivery/availability ensues. As such, cardiac output (SV x HR) must increase to compensate. Consider whether the patient regularly takes cardioactive medications such as beta blockers which will depress this clinical manifestation.
- Blood pressure may become elevated or remain within the patient’s normal range during the early stages of sepsis, as patients often initially enter a hyper-dynamic state. This may be further evidenced by bounding pulses or a widened pulse pressure if invasive blood pressure monitoring is in situ. Over time, compensatory mechanisms fail, and the patients’ blood pressure drops as they begin to approach septic shock (which will also contribute to a tachycardic response as the body attempts to compensate for the loss in vascular tone).
- Confusion or altered mental state is an indication of inadequate perfusion. Utilise family or carers in assessing this, as the patient may have altered cognition at a baseline e.g. if taking psychotropic medications, PHx dementia etc.
- Decreased urine output is a sensitive indicator of distal organ perfusion. As renal perfusion becomes compromised, the renin-angiotensin aldosterone system activates to increase sodium resorption at the distal tubule as a means of preventing fluid depletion in an already poor perfusion state.
- Skin often presents in a manner consistent with the patient’s vasoplegic state. The patient may feel hot to touch, with a hyper-brisk capillary refill time and may become peripherally oedematous as fluid begins to enter the interstitial space. In the later stages of sepsis/septic shock, skin can become cold, clammy, mottled and shut down.
- Lactate is often readily available as a point of care test in critical care environments and is a key biochemical marker for cellular dysfunction. As perfusion fails and metabolic demand increases, cells enter an anaerobic mode of metabolism which produces lactic acid as a by-product.
- Recent investigations for review/reassessment may include:
Pathology:
- Recent liver/renal function tests
- C-reactive protein
- Platelet count
- White cell / neutrophil count
Microbiology:
- Sputum
- Urine
- Bloods
- Wounds
- Invasive devices
Radiology:
- Chest x-ray if respiratory source is suspected
- CT if abdominal source is suspected
There is an emphasis on critical thinking by clinicians in current literature, as each of these criteria in isolation have low specificity for sepsis (National Institute for Health and Care Excellence, 2016). It is pivotal that skilled professionals ask the question “could this be sepsis?” while drawing on multiple data sources to form a clinical diagnosis (National Institute for Health and Care Excellence, 2016). In addition to the above clinical red flags, it is important to consider the patients’ history to aid in identifying a source of infection, which will require a thorough examination and laboratory testing (National Institute for Health and Care Excellence, 2016).
Clinical Challenges and Priorities
As with any medical emergency, an ABCDE approach should be utilised to stabilise the critically unwell patient. Commence BLS/ALS according to your skillset/training as indicated.
Airway:
- Consider the need to secure the airway in obtunded / critically hypoxaemic patients.
- Basic interventions: airway manoeuvres/adjuncts; suction; rescue position.
Breathing:
- Optimise oxygen availability: supplemental high flow O2 via NRB
- Provide ventilatory support as indicated
- Consider a respiratory source of infection:
- Auscultate lung fields
- obtain sputum samples
Circulation:
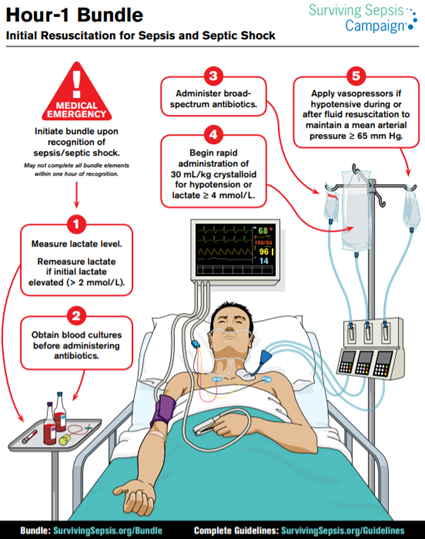
- Fluid resuscitation is a key component in the management of sepsis.
- Obtain wide-bore IV access (consider drawing blood cultures at this time)
- Surviving sepsis guidelines recommend 30ml/kg of crystalloid to target a MAP >65 mmHg, SBP >90 mmHg, or lactate <2 mmol/L.
- Vasopressor support
- Metaraminol can be administered peripherally pending central venous access
- Once central venous access is gained, consider changing vasopressor support to Noradrenaline, as Metaraminol works by releasing noradrenaline from sympathetic nerve endings and may deplete endogenous stores.
Disability:
- Monitor neurological state
- Assess for meningism, photophobia, or clonus as potential indicators of CNS infection
- Correct metabolic derangements:
- Noradrenaline can induce gluconeogenesis and exacerbate hyperglycamia among non-diabetic patients – monitor BGL and treat according to local procedures
- There is insufficient evidence to support routine bicarbonate administration in acidotic patients, however this may be a consideration in severe acidosis and can be discussed with senior medical practitioners.
Exposure:
- Necessary for identifying other potential sources e.g., necrotic pressure injury in diabetic patients, track marks in IVDU may indicate endocarditis.
With regards to general management of the septic patients, the Surviving Sepsis Campaign published several “bundles” to prompt the timely implementation of priority interventions (Rhodes et al., 2017)
The Nurses Role
The Nurse plays a pivotal role in the identification and management of septic patients. With Nurses working in such regular and close contact with patients, it is likely that they will be among the first to recognise the signs of sepsis. (Robson & Daniels, 2008).
Key considerations for the primary nurse include:
Thorough assessment utilising an ABCDE and/or head to toe approach as indicated, with frequent reassessment to identify signs of deterioration and effectiveness of interventions.
- Further to physical examination, it is important that full vital signs, urine output, mental status, lactate, and fluid balance are closely monitored.
Initiation of sepsis pathways depending on local policies and procedures (Ferguson et al., 2019).
- Many organisations have developed nurse-initiated sepsis pathways where Nurses can initiate investigations and treatment yo streamline the management of patients who meet specific sepsis criteria.
- Nurse initiated pathways may include but are not limited to:
- Ordering blood tests such as lactate, FBE, UEC, Coags, LFT and blood cultures
- Ordering chest x-ray
- Initiating an initial fluid bolus in the hypotensive patient
- Initiating broad spectrum antibiotics (particularly in patients who present with febrile neutropenia)
It is important to conform to your organisations policies and procedures so as not to breach scope of practice when independently initiating investigations and therapeutic interventions.
Nurse advocacy is paramount in the management of any patient, especially as their condition deteriorates. Even if there are no guidelines in your organisation to enable a nurse-initiated response, there is immeasurable value in remaining informed about current evidence-based clinical practice guidelines, as well as organisational policies and procedures to contribute to clinical decision making as the patients advocate. In doing so, the nurse can:
- Expedite treatment reaching the patient in a timely manner pending a medical response by having pre-emptively responded within their scope of practice. For example:
- Gaining wide-bore IV access
- Preparing and priming crystalloid fluid with a rapid infusion set
- Preparing necessary containers for standard bloods / culture collection
- Preparing necessary medications such as antibiotics and metaraminol
- Ensure that the medically prescribed therapies are in-line with best practice and appropriate for the patient. For example:
- Antibiotic selection – Organisational guidelines will often recommend different broad-spectrum antibiotics depending on whether the infection was acquired in the community versus in-hospital. If the responding medical practitioner prescribes an antibiotic recommended for community acquired infections and the nurse suspects a hospital acquired infection, they can only effectively advocate for their patient if they are aware of antibiotic selection protocols.
Above all else, it is vital that the nurse effectively escalates care if they suspect that the patient is deteriorating, or they do not feel that their patient is safe in the current environment or receiving the appropriate level of care. It is important to notify senior clinicians and consider early referral to critical care services.
References
Bruce, H. R., Maiden, J., Fedullo, P. F., & Kim, S. C. (2015). Impact of nurse-initiated ED sepsis protocol on compliance with sepsis bundles, time to initial antibiotic administration, and in-hospital mortality. Journal of Emergency Nursing, 41(2), 130-137. 10.1016/j.jen.2014.12.007
Cawcutt, K. A., & Peters, S. G. (2014). Severe sepsis and septic shock: Clinical overview and update on management. Mayo Clinic, 89(11), 1572-1578. 10.1016/j.mayocp.2014.07.009
Chousterman, B. G., Swirski, F. K., & Weber, G. F. (2017). Cytokine storm and sepsis disease pathogenesis. Seminars in Immunopathology, 39(5), 517-528. 10.1007/s00281-017-0639-8
Ferguson, A., Coates, D. E., Osborn, S., Christopher, C., & Williams, B. (2019). Early, nurse-directed sepsis care. American Journal of Nursing, 119(1), 52-58. 10.1097/01.NAJ.0000552614.89028.d6
the George Institute for Global Health. (2021). Review of trigger tools to support the early identification of sepsis in healthcare settings. Australian Commission on Safety and Quality in Health Care. https://www.safetyandquality.gov.au/publications-and-resources/resource-library/review-trigger-tools-support-early-identification-sepsis-healthcare-settings
Khojandi, A., & Tansakul, V. (2018). Prediction of sepsis and in-hospital mortality using electronic health records. Methods of Information in Medicine, 57(4), 185-193. 10.3414/ME18-01-0014
Li, J., Chen, J., & Kirsner, R. (2007). Pathophysiology of acute wound healing. Clinics in Dermatology, 25(1), 9-18. 10.1016/j.clindermatol.2006.09.007
Marik, P. E., & Taeb, A. M. (2017). SIRS, qSOFA and new sepsis definition. Journal of Thoracic Disease, 9(4), 943-945. 10.21037/jtd.2017.03.125
National Institute for Health and Care Excellence. (2016). Sepsis: recognition, diagnosis and early management.
Rhodes, A., Evans, L. E., Alhazzani, W., Levy, M. L., Antonelli, M., Ferrer, R., Kumar, A., Sevransky, J., Sprung, C. L., & Dellinger, P. R. (2017). Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Critical Care Medicine, 45(3), 486-552. 10.1097/CCM.0000000000002255
Robson, P. W., & Daniels, R. (2008). The Sepsis Six: helping patients to survive sepsis. British Journal of Nursing, 17(1), 16-21.
Usman, O. A., Usman, A. A., & Ward, M. A. (2019). Comparison of SIRS, qSOFA, and NEWS for the early identification of sepsis in the Emergency Department. the American Journal of Emergency Medicine, 37(8), 1490-1497. 10.1016/j.ajem.2018.10.058






You must be logged in to post a comment.